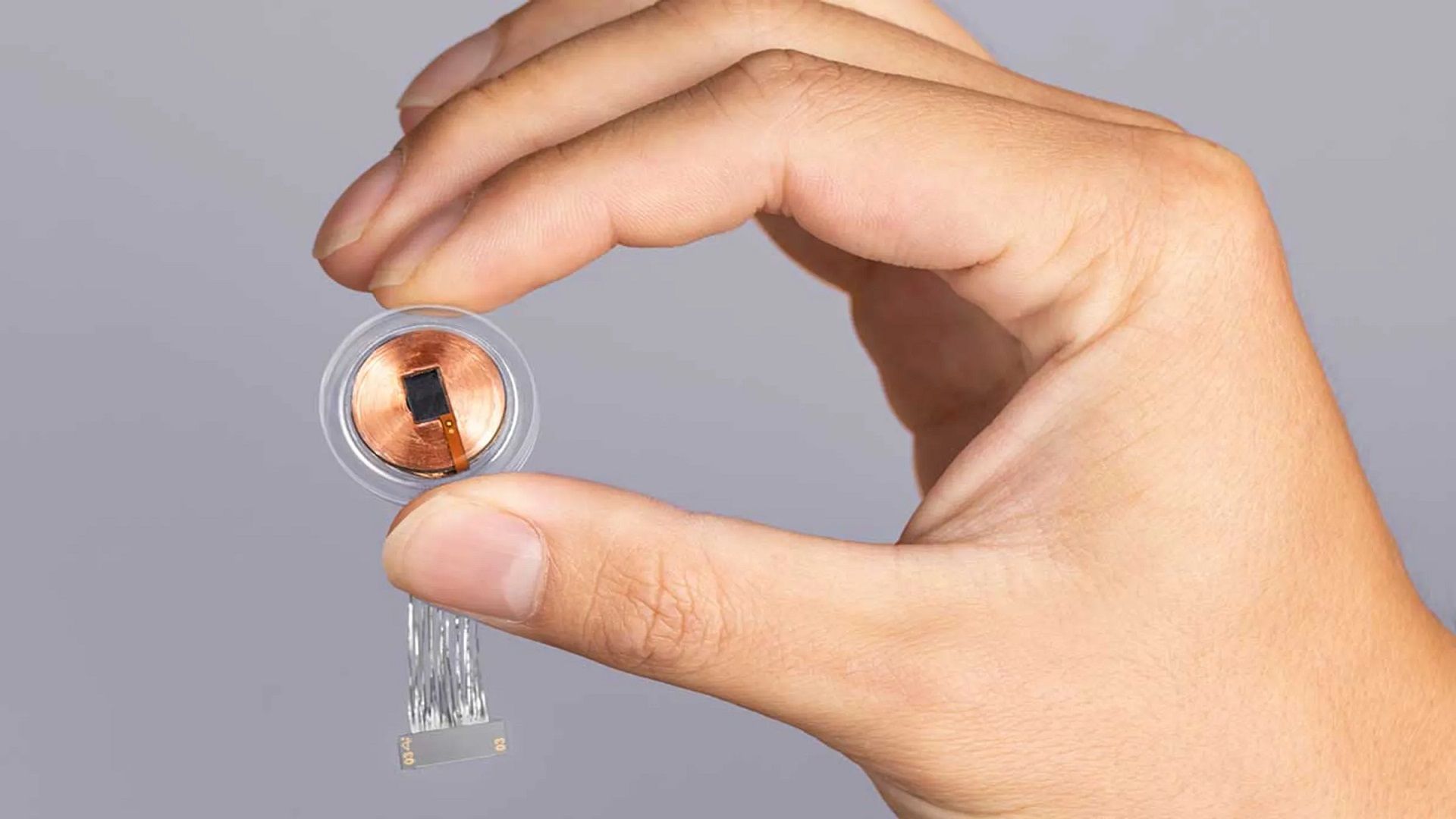
Neuralink human trials have been one of the biggest struggles of the company moving forward with its revolutionary technology. In a remarkable development, Elon Musk’s brain-computer interface company, Neuralink, has received regulatory clearance from the Food and Drug Administration (FDA) to proceed with its highly anticipated first-in-human clinical trial. The approval marks a significant milestone for Neuralink, following previous challenges in gaining regulatory endorsement for its groundbreaking technology.
Neuralink announced the FDA’s nod via a tweet, acknowledging the approval as an important initial step towards realizing the potential of their technology in helping a multitude of people. While the company did not provide specific details about the objectives of the study, they confirmed that recruitment had not yet begun, promising to release more information in the near future. Nevertheless, a patient registry page has been published on the company’s website, including a link for the application.
We are excited to share that we have received the FDA’s approval to launch our first-in-human clinical study!
This is the result of incredible work by the Neuralink team in close collaboration with the FDA and represents an important first step that will one day allow our…
— Neuralink (@neuralink) May 25, 2023
What do Neuralink human trials implicate for the future of neuroscience?
Elon Musk envisions Neuralink’s brain implants as a potential panacea for a range of conditions, including obesity, autism, depression, and schizophrenia. Furthermore, he believes the technology could unlock capabilities such as web browsing and even telepathy. Musk’s confidence in the safety of the devices was demonstrated when he publicly expressed his willingness to have them implanted in his own children, garnering significant attention.
However, Neuralink has faced setbacks in its journey toward human trials. Musk had previously made multiple predictions since 2019 about commencing Neuralink human trials, but the company only sought FDA approval in early 2022, which was initially rejected. Neuralink even began accepting applications for trial volunteers in December 2022.
According to sources within the company, the FDA had raised several concerns that needed to be addressed before granting approval. These concerns included the device’s lithium battery, the potential migration of wires within the brain, and the safe extraction of the device without causing harm to brain tissue.
Neuralink, founded in 2016, has also faced scrutiny from federal authorities. In May, U.S. lawmakers urged regulators to investigate potential misconduct in Neuralink’s treatment of animal test subjects, which the company vehemently denies.
Additionally, the Department of Transportation is conducting a separate probe to determine whether Neuralink violated regulations by transporting contaminated devices removed from monkeys without proper containment measures. Moreover, the U.S. Department of Agriculture’s Office of Inspector General is investigating Neuralink for potential animal welfare violations and evaluating the USDA’s oversight of the company.
While the company deals with these ongoing investigations, the FDA’s approval for the first Neuralink human trials solidifies the company’s position in the race to develop advanced brain-computer interfaces. It’s worth noting that Neuralink is not the only player in this field, as Synchron, another brain-computer interface company, received FDA approval to commence trials in the United States in 2021. Synchron’s breakthroughs, including the first U.S. brain-computer implant announced in July of that year, have contributed to the growing body of research and understanding in this domain.
If you’re considering participating in the Neuralink human trials, it’s important to note that recruitment has not yet commenced. Neuralink has assured interested individuals that more information will be provided soon, allowing them to make informed decisions. Yet, you can still refer to the patient registry page on the website.
The FDA’s approval for the first Neuralink human trials represents a significant leap forward in the realm of brain-computer interfaces. As the trial progresses and yields valuable insights, the potential applications in medicine and human-machine interaction become increasingly fascinating. Neuralink’s journey to revolutionize the field of neuroscience is undoubtedly one to watch closely as it unfolds, with the potential to reshape our understanding of the brain and open doors to new possibilities.





