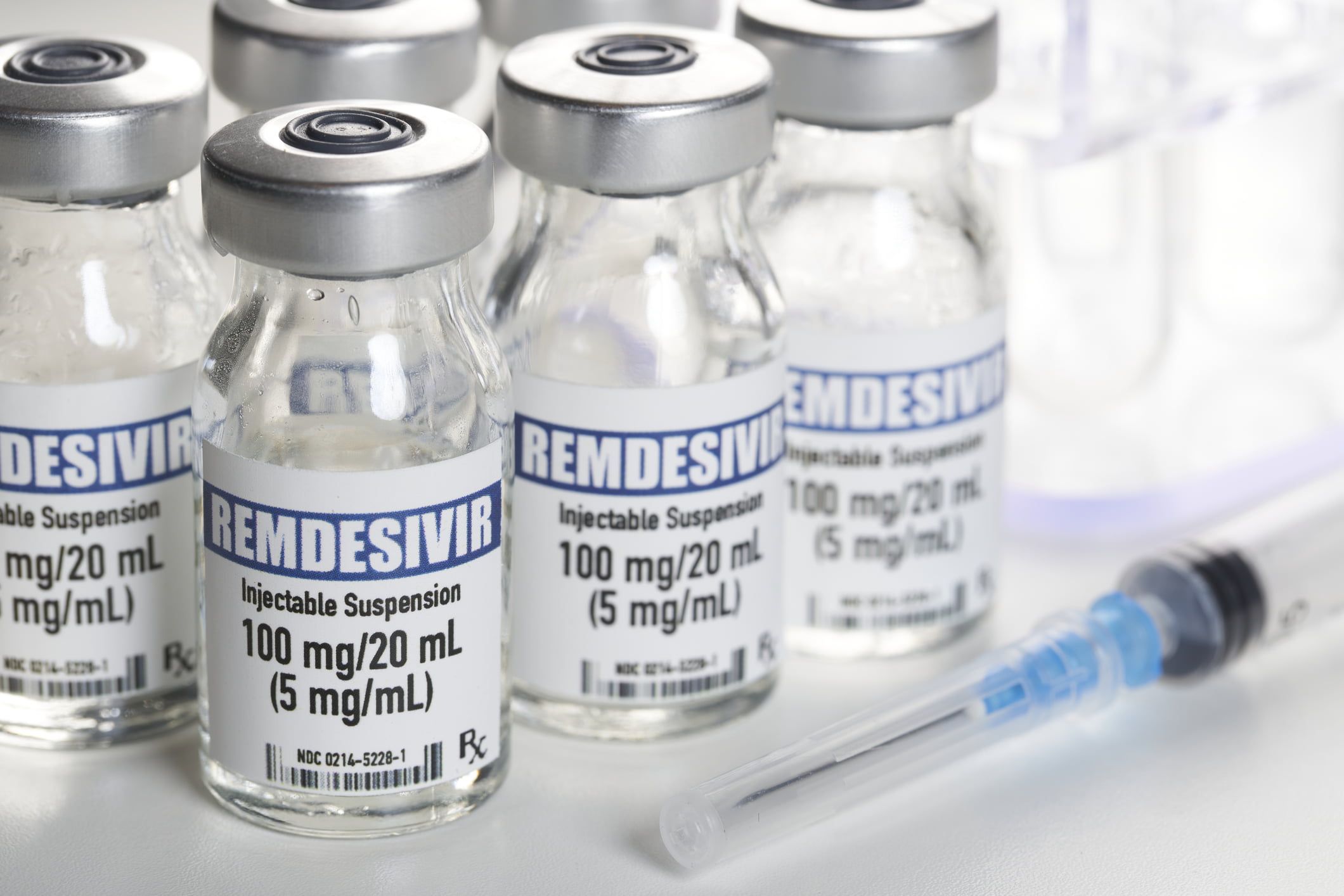
The US Food and Drug Administration approves the antiviral drug Remdesivir for the treatment of COVID-19 patients. Most prominent patient so far: Trump.
Remdesivir seems to be the only drug that is approved for COVID-19 treatment
The U.S. Food and Drug Administration (FDA) approves the antiviral drug Remdesivir as the first and so far only drug for the treatment of COVID-19 patients. This was announced on Thursday.
It is administered intravenously to patients with severe courses of disease who need to be hospitalized. The drug reduces the average recovery time of patients from 15 to 10 days, according to a large-scale study conducted by the U.S. National Institutes of Health.
President Trump treated with Remdesivir
The drug from the U.S. manufacturer Gilead has so far only had a provisional emergency approval. The most prominent patient treated with Remdesivir is US President Donald Trump.
The drug, which is known as Veklury in Gilead, had been under emergency approval since the spring and is now the first drug to receive full approval in the U.S. for the treatment of corona patients.
It is approved for people aged twelve years and older and weighing at least 40 kilograms who require inpatient treatment, Gilead said. Remdesivir is said to inhibit the ability of the virus to replicate in the body.
WHO: Remdesivir’s benefit is not clear
However, the World Health Organization (WHO) announced last week that several potential corona drugs that had been tested in worldwide test series had shown little or no benefit. Remdesivir was among them was.
However, the data from the WHO-coordinated solidarity study had not yet been published in a peer-reviewed journal and had not been reviewed by scientists.